About Us
Discover our mission, partners, governance and more.
Our mission
Increasing European and global capacity to prevent and respond to infectious threats.
The ISIDORe project assembles the largest and most diverse research and service-providing instrument to study infectious diseases in Europe, with expertise from structural biology to clinical trials.
By giving scientists access to the whole extent of our state-of-the-art facilities, cutting-edge services, advanced equipment and expertise, in an integrated way and with a common goal, we will accelerate the generation of new knowledge and intervention tools to enhance Europe’s capacity for controlling (re)emerging and epidemic infectious diseases, starting with the COVID-19 pandemic.
OUR PARTNERS
Under the umbrella of 17 major European life sciences research infrastructures and infectious diseases networks coordinated by ERINHA, we bring together 154 research entities and organizations providing services to advance research on epidemic-prone diseases.
 Visit our partner BBMRI
Visit our partner BBMRI
 Visit our partner Eatris
Visit our partner Eatris
 Visit our partner Elixir
Visit our partner Elixir
 Visit our partner Embrc
Visit our partner Embrc
 Visit our partner EU-Openscreen
Visit our partner EU-Openscreen
 Visit our partner Euro-Bioimaging
Visit our partner Euro-Bioimaging
 Visit our partner Eva-g
Visit our partner Eva-g
 Visit our partner Infrafrontier
Visit our partner Infrafrontier
 Visit our partner Infravec
Visit our partner Infravec
 Visit our partner Instruct
Visit our partner Instruct
 Visit our partner Mirri
Visit our partner Mirri
 Visit our partner Sonar-Global
Visit our partner Sonar-Global
 Visit our partner Transvac
Visit our partner Transvac
 Visit our partner Vetbionet
Visit our partner Vetbionet

ISIDORe by numbers
A large-scale multidisciplinary consortium addressing the users’ needs
Funding, million euros
21
Services
300+
Partners
154
Countries involved
32
Duration, years
3

WHAT WE DO
ISIDORe supports scientists and their research on epidemic- and pandemic-prone pathogens, the development of medical countermeasures, with the aim of increasing resilience in the face of epidemics in Europe and globally.
Free transnational access to research resources, services and expertise
Transnational access (TNA) calls for proposals
The provision of research services to researchers is organised through calls for proposals and the selection of research projects to access ISIDORe resources for free.
Learn about Transnational Access and see our open calls for proposals.
Development of new services
We conduct research activities with an immediate impact on the quality, diversity and relevance of our services to offer the most advanced, innovative and cutting-edge catalogue of services possible.
You did not find the service you need in our catalogue? Please let us know by filling out the dedicated form.
QUALITY CRITERIA
We have mapped, through a comprehensive survey and intensive desk research, applicable quality and safety relevant international and national laws, policies, international and European standards, guidelines as well as scientific recommendations and best practices for biospecimen handling to improve and facilitate these procedures across ISIDORe partners.
International Laws
To facilitate a mutual understanding between all services provided by ISIDORe partners an overview of applicable international laws and regulations for infectious disease research services was compiled. These include safety regulations, regulations for the transport of biospecimens, regulations on the handling of genetically modified organisms (GMOs), animal welfare, data protection regulations, ethics, the conduct of clinical trials and the handling of medical products.
NATIONAL LAWS
National laws for handling (potentially) infectious materials serve multiple purposes. They are primarily aimed at protecting public health and preventing the spread of infectious diseases. ISIDORe partners providing infectious disease research services must ensure that they comply with all relevant legal requirements (permits and agreements). In addition to the relevance for the service providers the overview of national laws and regulations enables users of ISIDORe services to identify applicable requirements and to ensure that they are acting in accordance with the respective laws in each country.
Overview of relevant ISIDORe Work packages and the required and/or provided biospecimens for offered services
To leverage the full potential of the ISIDORe consortium and thus to realise more ambitious and impactful research projects in the field of infectious disease research, an interdisciplinary approach is necessary.
International Standards, best practices, guidelines and recommendations FOR BIOSPECIMENS
International standards, best practices, guidelines and scientific recommendations are of paramount importance for ISIDORe partners, which provide services and biospecimens for infectious disease research. Adherence to these international standards increases the comparability and consistency of research data and results and enables more effective collaboration among scientists from different countries and institutions. These standards serve to maintain high-quality research standards, from provision of reference samples, collection and processing of biospecimens to data analysis and reporting. This improves the reliability of data and the reproducibility of biomedical research. Similarly, compliance with these standards is critical to protecting the rights and welfare of participants (and/or animals), better navigating the various regulatory environments, streamlining approval processes, and thereby maintaining the trust of the public and regulators.
International Standards, best practices, guidelines and recommendations FOR infectious disease RESEARCH services
In this section an overview of all infectious disease research services offered by ISIDORe service partners, a general overview of relevant international standards and scientific recommendations as well as specific minimum requirements for each service provider to use their services is presented.
ISIDORe governance
ISIDORe is coordinated by the European Research Infrastructure on Highly Pathogenic Agents (ERINHA).
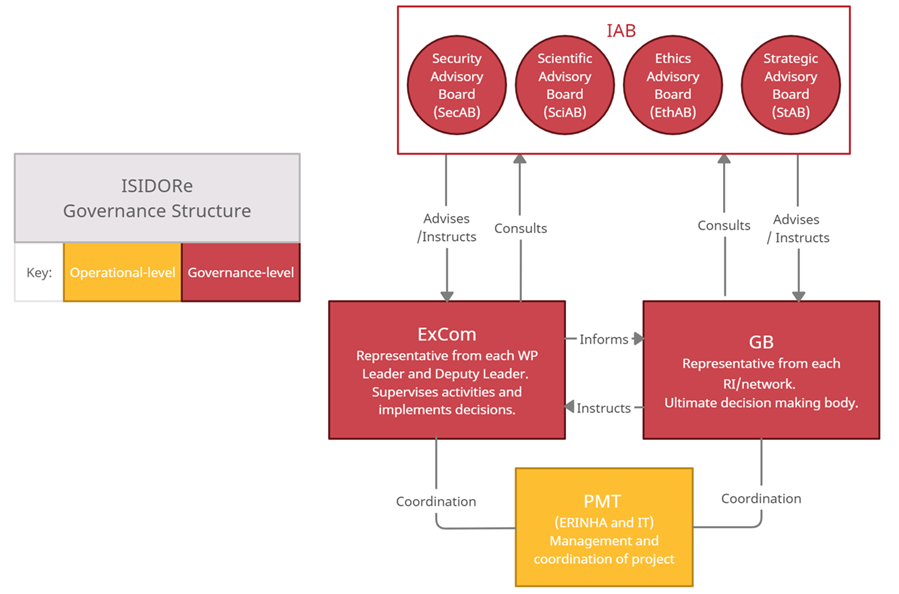
Governing board (gb)
The Governing Board is made up of representatives from each of the 17 Research Infrastructures and Networks of the ISIDORe consortium.
Executive committee (excom)
The ISIDORe Executive Committee supervises the execution of the action and implements the decisions made by the GB. The ExCom is made up of the leaders of each Work Package in the project.
Project management team
The project management team (PMT) is composed of staff from ERINHA and Inserm-Transfert who monitor project progress and manage the day-to-day functions of the project.
For any information related to the project contact us at: contact(at)isidore-project.eu.
Independent Advisory Board
The IAB is a multi-purpose board with four distinct sections, made up of the appropriate experts to support the project in strategy (StAB), science (SciAB), ethics (EthAB), and security (SecAB). The IAB acts as an interface with the relevant international agencies, initiatives, projects and individual stakeholders.
Strategic Advisory Board (StAB)
The goal of the StAB is to align ISIDORe’s activities with the priorities of actors of pandemic management and preparedness, funders, regulatory decision-makers, the pharmaceutical industry, etc.
- Coalition for Epidemic Preparedness Innovations (CEPI)
- European Centre for Disease Prevention and Control (ECDC)
- European Commission Unit Combating Diseases, DG R&I
- European Federation for Pharmaceutical Industries and Associations (EFPIA)
- European Health Emergency preparedness and Response Authority (HERA)
- European Medicines Agency (EMA)
- Global Research Collaboration for Infectious Disease Preparedness (GloPID-R)
- UN Food and Agriculture Organisation (FAO)
- World Health Organisation (WHO)
- World Organisation for Animal Health (OIE)
Scientific Advisory Board (SciAB)
The SciAB is made up of experts representing the user community across the range of areas of scientific research made available through the ISIDORe project. The SciAB will provide instructions on the priority research topics to be addressed to improve or extend ISIDORe’s catalogue of services.
Ethics Advisory Board (EthAB)
The EthAB includes experts in clinical studies, animal welfare, and data protection, who will monitor ISIDORe’s compliance with all relevant ethical regulations and standards pertaining to the activities of the project.
Security Advisory Board (SecAB)
he security concerns potentially raised by the project. The SecAB will evaluate the sensitivity of the information contained in project deliverables and propose measures for preventing the misuse of such information.

Outcomes & Impact
Outcomes
We…
- Advance research on epidemic-prone diseases
- Increase epidemic preparedness and responsiveness
- Support the development of medical countermeasures against epidemic-prone pathogens
- Share research data emerging from access provision activities according to FAIR principles
Impact
ISIDORe is a unique European integrated large-scale research infrastructure capacity for conducting research on any epidemic-prone pathogen, including SARS-CoV-2. It will be a powerful instrument at the disposal of the EU, and will impact its capacity to identify, characterise and mitigate the effects of any epidemic-prone pathogens.
ISIDORe aims to enable ground-breaking discoveries and the development of new medical countermeasures with high impact on health security and society.
Trainings
The ISIDORe partners and their collaborators offer a broad portfolio of training opportunities that are consistent with, but not limited to, the ISIDORe objective of enhancing European and global capacity to prevent and respond to infectious threats.